Stainless steel
| Iron alloy phases |
|---|
|
| Steel classes |
|
| Other iron-based materials |
|
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5[1] or 11% chromium content by mass.[2]
Stainless steel does not corrode, rust or stain with water as ordinary steel does, but despite the name it is not fully stain-proof.[3] It is also called corrosion-resistant steel or CRES when the alloy type and grade are not detailed, particularly in the aviation industry. There are different grades and surface finishes of stainless steel to suit the environment the alloy must endure. Stainless steel is used where both the properties of steel and resistance to corrosion are required.
Stainless steel differs from carbon steel by the amount of chromium present. Unprotected carbon steel rusts readily when exposed to air and moisture. This iron oxide film (the rust) is active and accelerates corrosion by forming more iron oxide. Stainless steels contain sufficient chromium to form a passive film of chromium oxide, which prevents further surface corrosion and blocks corrosion from spreading into the metal's internal structure.[4]
Passivation only occurs if the proportion of chromium is high enough.
Contents |
History
A few corrosion-resistant iron artifacts survive from antiquity. A famous example is the Iron Pillar of Delhi, erected by order of Kumara Gupta I around AD 400. Unlike stainless steel, however, these artifacts owe their durability not to chromium but to their high phosphorus content, which, together with favorable local weather conditions, promotes the formation of a solid protective passivation layer of iron oxides and phosphates, rather than the non-protective cracked rust layer that develops on most ironwork.
The corrosion resistance of iron-chromium alloys was first recognized in 1821 by French metallurgist Pierre Berthier, who noted their resistance against attack by some acids and suggested their use in cutlery. Metallurgists of the 19th century were unable to produce the combination of low carbon and high chromium found in most modern stainless steels, and the high-chromium alloys they could produce were too brittle to be practical.
In the late 1890s Hans Goldschmidt of Germany developed an aluminothermic (thermite) process for producing carbon-free chromium. Between 1904 and 1911 several researchers, particularly Leon Guillet of France, prepared alloys that would today be considered stainless steel.
Friedrich Krupp Germaniawerft built the 366-ton sailing yacht Germania featuring a chrome-nickel steel hull in Germany in 1908.[6] In 1911, Philip Monnartz reported on the relationship between chromium content and corrosion resistance. On October 17, 1912, Krupp engineers Benno Strauss and Eduard Maurer patented austenitic stainless steel as ThyssenKrupp Nirosta.[7]
Similar developments were taking place contemporaneously in the United States, where Christian Dantsizen and Frederick Becket were industrializing ferritic stainless steel. In 1912, Elwood Haynes applied for a US patent on a martensitic stainless steel alloy, which was not granted until 1919.[8]
Also in 1912, Harry Brearley of the Brown-Firth research laboratory in Sheffield, England, while seeking a corrosion-resistant alloy for gun barrels, discovered and subsequently industrialized a martensitic stainless steel alloy. The discovery was announced two years later in a January 1915 newspaper article in The New York Times.[5] The metal was later marketed under the 'Staybrite' brand by Firth Vickers in England and was used for the new entrance canopy for the Savoy Hotel in London in 1929.[9]
Brearley applied for a US patent during 1915 only to find that Haynes had already registered a patent. Brearley and Haynes pooled their funding and with a group of investors formed the American Stainless Steel Corporation, with headquarters in Pittsburgh, Pennsylvania.[10] In the beginning stainless steel was sold in the US under different brand names like 'Allegheny metal' and 'Nirosta steel'. In 1929 before the Great Depression hit, over 25,000 tons of stainless steel was manufactured and sold in the US.[11]
Properties
High oxidation-resistance in air at ambient temperature is normally achieved with additions of a minimum of 13% (by weight) chromium, and up to 26% is used for harsh environments.[12] The chromium forms a passivation layer of chromium(III) oxide (Cr2O3) when exposed to oxygen. The layer is too thin to be visible, and the metal remains lustrous. The layer is impervious to water and air, protecting the metal beneath. Also, this layer quickly reforms when the surface is scratched. This phenomenon is called passivation and is seen in other metals, such as aluminium and titanium. Corrosion-resistance can be adversely affected if the component is used in a non-oxygenated environment, a typical example being underwater keel bolts buried in timber.
When stainless steel parts such as nuts and bolts are forced together, the oxide layer can be scraped off, causing the parts to weld together. When disassembled, the welded material may be torn and pitted, an effect known as galling. This destructive galling can be best avoided by the use of dissimilar materials for the parts forced together, for example bronze and stainless steel, or even different types of stainless steels (martensitic against austenitic), when metal-to-metal wear is a concern. Nitronic alloys reduce the tendency to gall through selective alloying with manganese and nitrogen. In addition, threaded joints may be lubricated to prevent galling.
Applications
Stainless steel’s resistance to corrosion and staining, low maintenance and familiar lustre make it an ideal material for many applications. There are over 150 grades of stainless steel, of which fifteen are most commonly used. The alloy is milled into coils, sheets, plates, bars, wire, and tubing to be used in cookware, cutlery, hardware, surgical instruments, major appliances, industrial equipment (for example, in sugar refineries) and as an automotive and aerospace structural alloy and construction material in large buildings. Storage tanks and tankers used to transport orange juice and other food are often made of stainless steel, because of its corrosion resistance and antibacterial properties. This also influences its use in commercial kitchens and food processing plants, as it can be steam-cleaned and sterilized and does not need paint or other surface finishes.
Stainless steel is used for jewellery and watches with 316L being the type commonly used for such applications. It can be re-finished by any jeweler and will not oxidize or turn black.
Some firearms incorporate stainless steel components as an alternative to blued or parkerized steel. Some handgun models, such as the Smith & Wesson Model 60 and the Colt M1911 pistol, can be made entirely from stainless steel. This gives a high-luster finish similar in appearance to nickel plating. Unlike plating, the finish is not subject to flaking, peeling, wear-off from rubbing (as when repeatedly removed from a holster), or rust when scratched.
Some automotive manufacturers use stainless steel as decorative highlights in their vehicles.
Architectural
Stainless steel is used for buildings for both practical and aesthetic reasons. Stainless steel was in vogue during the art deco period. The most famous example of this is the upper portion of the Chrysler Building (pictured). Some diners and fast-food restaurants use large ornamental panels and stainless fixtures and furniture. Because of the durability of the material, many of these buildings retain their original appearance.
The forging of stainless steel has given rise to a fresh approach to architectural blacksmithing in recent years.
Type 316 stainless is used on the exterior of both the Petronas Twin Towers and the Jin Mao Building, two of the world's tallest skyscrapers.[14]
The Parliament House of Australia in Canberra has a stainless steel flagpole weighing over 220 tons (200 metric tonnes).
The aeration building in the Edmonton Composting Facility, the size of 14 hockey rinks, is the largest stainless steel building in North America.
Bridges
- Minorca Road Bridge (Spain) is the first stainless steel road bridge.
- Sant Fruitos Pedestrian Bridge (Catalonia, Spain), arch pedestrian bridge.
- Padre Arrupe Bridge (Bilbao, Spain) links the Guggenheim museum to the University of Deusto.[15]
Monuments and sculptures
- The Unisphere, constructed as the theme symbol of the 1964-5 World's Fair in New York City, is the world's largest globe-shaped structure.
- The Gateway Arch (pictured) is clad entirely in stainless steel: 886 tons (804 metric tonnes) of 0.25 in (6.4 mm) plate, #3 finish, type 304 stainless steel.[16]
- The United States Air Force Memorial has an austenitic stainless steel structural skin.
- The Atomium in Brussels, Belgium was renovated with stainless-steel cladding in a renovation completed in 2006; previously the spheres and tubes of the structure were clad in aluminium.
- The Cloud Gate sculpture by Anish Kapoor, in Chicago US.
- The Sibelius monument in Helsinki, Finland, is made entirely of stainless steel tubes.
Other
- The DeLorean DMC-12 automobile used stainless steel body panels over a glass-reinforced plastic monocoque.
- Passenger rail cars have commonly been manufactured using corrugated stainless steel panels (for additional structural strength). One notable example was the early Pioneer Zephyr.
Recycling and reuse
Stainless steel is 100% recyclable. An average stainless steel object is composed of about 60% recycled material of which approximately 40% originates from end-of-life products and about 60% comes from manufacturing processes.[17] According to the International Resource Panel's Metal Stocks in Society report, the per capita stock of stainless steel in use in society is 80–180kg in more developed countries and 15kg in less-developed countries.
There is a secondary market that recycles usable scrap for many stainless steel markets. The product is mostly coil, sheet and blanks. This material is purchased at a less-than-prime price and sold to commercial quality stampers and sheet metal houses. The material may have scratches, pits and dents but is made to the current specifications.
Types of stainless steel
There are different types of stainless steels: when nickel is added, for instance, the austenite structure of iron is stabilized. This crystal structure makes such steels virtually non-magnetic and less brittle at low temperatures. For greater hardness and strength, more carbon is added. With proper heat treatment, these steels are used for such things as razor blades, cutlery, and tools.
Significant quantities of manganese have been used in many stainless steel compositions. Manganese preserves an austenitic structure in the steel as does nickel, but at a lower cost.
Stainless steels are also classified by their crystalline structure:
- Austenitic, or 300 series, stainless steels make up over 70% of total stainless steel production. They contain a maximum of 0.15% carbon, a minimum of 16% chromium and sufficient nickel and/or manganese to retain an austenitic structure at all temperatures from the cryogenic region to the melting point of the alloy. A typical composition of 18% chromium and 10% nickel, commonly known as 18/10 stainless, is often used in flatware. 18/0 and 18/8 are also available.
- Superaustenitic stainless steels, such as alloy AL-6XN and 254SMO, exhibit great resistance to chloride pitting and crevice corrosion because of high molybdenum content (>6%) and nitrogen additions, and the higher nickel content ensures better resistance to stress-corrosion cracking versus the 300 series. The higher alloy content of superaustenitic steels makes them more expensive. Other steels can offer similar performance at lower cost and are preferred in certain applications, for example ASTM A387 is used in pressure vessels but is a low alloy carbon steel with a chromium content of 0.5% to 9%. [18] Low-carbon versions, for example 316L or 304L, are used to avoid corrosion problems caused by welding. Grade 316LVM is preferred where biocompatibility is required (such as body implants and piercings).[19] The "L" means that the carbon content of the alloy is below 0.03%, which reduces the sensitization effect (precipitation of chromium carbides at grain boundaries) caused by the high temperatures involved in welding.
- Ferritic stainless steels generally have better engineering properties than austenitic grades, but have reduced corrosion resistance, because of the lower chromium and nickel content. They are also usually less expensive. They contain between 10.5% and 27% chromium and very little nickel, if any, but some types can contain lead. Most compositions include molybdenum; some, aluminium or titanium. Common ferritic grades include 18Cr-2Mo, 26Cr-1Mo, 29Cr-4Mo, and 29Cr-4Mo-2Ni. These alloys can be degraded by the presence of
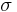 chromium, an intermetallic phase which can precipitate upon welding.
chromium, an intermetallic phase which can precipitate upon welding.
- Martensitic stainless steels are not as corrosion-resistant as the other two classes but are extremely strong and tough, as well as highly machinable, and can be hardened by heat treatment. Martensitic stainless steel contains chromium (12–14%), molybdenum (0.2–1%), nickel (less than 2%), and carbon (about 0.1–1%) (giving it more hardness but making the material a bit more brittle). It is quenched and magnetic.
- Precipitation-hardening martensitic stainless steels have corrosion resistance comparable to austenitic varieties, but can be precipitation hardened to even higher strengths than the other martensitic grades. The most common, 17-4PH, uses about 17% chromium and 4% nickel. The Lockheed-Martin Joint Strike Fighter is the first aircraft to use a precipitation-hardenable stainless steel—Carpenter Custom 465—in its airframe.
- Duplex stainless steels have a mixed microstructure of austenite and ferrite, the aim usually being to produce a 50/50 mix, although in commercial alloys the ratio may be 40/60. Duplex stainless steels have roughly twice the strength compared to austenitic stainless steels and also improved resistance to localized corrosion, particularly pitting, crevice corrosion and stress corrosion cracking. They are characterized by high chromium (19–32%) and molybdenum (up to 5%) and lower nickel contents than austenitic stainless steels.
- The properties of duplex stainless steels are achieved with an overall lower alloy content than similar-performing super-austenitic grades, making their use cost-effective for many applications. Duplex grades are characterized into groups based on their alloy content and corrosion resistance.
- Lean duplex refers to grades such as UNS S32101 (LDX 2101), S32304, and S32003.
- Standard duplex is 22% chromium with UNS S31803/S32205 known as 2205 being the most widely used.
- Super duplex is by definition a duplex stainless steel with a pitting corrosion equivalent (PRE) > 40, where PRE = %Cr + 3.3x(%Mo + 0.5x%W) + 16x%N. Usually super duplex grades have 25% chromium or more and some common examples are S32760 (Zeron 100), S32750 (2507) and S32550 (Ferralium), although not all Ferralium grades are super duplex grades.
- Hyper duplex refers to duplex grades with a PRE > 48 and at the moment only UNS S32707 and S33207 are available on the market.
Comparison of standardized steels
| EN-standard
Steel no. k.h.s DIN |
EN-standard
Steel name |
SAE grade | UNS |
|---|---|---|---|
| 440A | S44002 | ||
| 1.4112 | X90CrMoV18 | 440B | S44003 |
| 1.4125 | 440C | S44004 | |
| 440F | S44020 | ||
| 1.4016 | X6Cr17 | 430 | S43000 |
| 1.4408 | G-X 6 CrNiMo 18-10 | 316 | |
| 1.4512 | X6CrTi12 | 409 | S40900 |
| 410 | S41000 | ||
| 1.4310 | X10CrNi18-8 | 301 | S30100 |
| 1.4318 | X2CrNiN18-7 | 301LN | N/A |
| 1.4307 | X2CrNi18-9 | 304L | S30403 |
| 1.4306 | X2CrNi19-11 | 304L | S30403 |
| 1.4311 | X2CrNiN18-10 | 304LN | S30453 |
| 1.4301 | X5CrNi18-10 | 304 | S30400 |
| 1.4948 | X6CrNi18-11 | 304H | S30409 |
| 1.4303 | X5CrNi18-12 | 305 | S30500 |
| X5CrNi30-9 | 312 | ||
| 1.4541 | X6CrNiTi18-10 | 321 | S32100 |
| 1.4878 | X12CrNiTi18-9 | 321H | S32109 |
| 1.4404 | X2CrNiMo17-12-2 | 316L | S31603 |
| 1.4401 | X5CrNiMo17-12-2 | 316 | S31600 |
| 1.4406 | X2CrNiMoN17-12-2 | 316LN | S31653 |
| 1.4432 | X2CrNiMo17-12-3 | 316L | S31603 |
| 1.4435 | X2CrNiMo18-14-3 | 316L | S31603 |
| 1.4436 | X3CrNiMo17-13-3 | 316 | S31600 |
| 1.4571 | X6CrNiMoTi17-12-2 | 316Ti | S31635 |
| 1.4429 | X2CrNiMoN17-13-3 | 316LN | S31653 |
| 1.4438 | X2CrNiMo18-15-4 | 317L | S31703 |
| 1.4362 | X2CrNi23-4 | 2304 | S32304 |
| 1.4462 | X2CrNiMoN22-5-3 | 2205 | S31803/S32205 |
| 1.4539 | X1NiCrMoCu25-20-5 | 904L | N08904 |
| 1.4529 | X1NiCrMoCuN25-20-7 | 1925hMo/6MO | N08926 |
| 1.4547 | X1CrNiMoCuN20-18-7 | 254SMO | S31254 |
Stainless steel grades
There are a number of different systems for grading stainless and other steels. The article on US SAE steel grades details a large number of grades with their properties.
Stainless steel in 3D printing
Some 3D printing providers have developed proprietary stainless steel sintering[20] blends for use in rapid prototyping. Currently available grades do not vary significantly in their properties.[21]
Stainless steel finishes
Standard mill finishes can be applied to flat rolled stainless steel directly by the rollers and by mechanical abrasives. Steel is first rolled to size and thickness and then annealed to change the properties of the final material. Any oxidation that forms on the surface (mill scale) is removed by pickling, and a passivation layer is created on the surface. A final finish can then be applied to achieve the desired aesthetic appearance.
- No. 0: Hot rolled, annealed, thicker plates
- No. 1: Hot rolled, annealed and passivated
- No. 2D: Cold rolled, annealed, pickled and passivated
- No. 2B: Same as above with additional pass-through highly polished rollers
- No. 2BA: Bright annealed (BA or 2R) same as above then bright annealed under oxygen-free atmospheric condition
- No. 3: Coarse abrasive finish applied mechanically
- No. 4: Brushed finish
- No. 5: Satin finish
- No. 6: Matte finish
- No. 7: Reflective finish
- No. 8: Mirror finish
- No. 9: Bead blast finish
- No. 10: Heat colored finish-wide range of electropolished and heat colored surfaces
See also
|
|
- SAE steel grades - List of grades and their respective properties.
References
- ^ "The Stainless Steel Family". http://www.worldstainless.org/NR/rdonlyres/B2617D50-73AE-4FAB-BDCD-88ABD7891B97/4933/TheStainlessSteelFamily.pdf. Retrieved 2009-11-12.
- ^ "Steel Glossary". American Iron and Steel Institute (AISI). http://www.steel.org/AM/Template.cfm?Section=Steel_Glossary2&CONTENTID=6426&TEMPLATE=/CM/HTMLDisplay.cfm. Retrieved October 21, 2008.
- ^ "Why is Stainless Steel Stainless?". http://www.stainless-online.com/why-stainless-steel-stainless.htm. Retrieved 2008-12-20..
- ^ Stainless Steels and Alloys: Why They Resist Corrosion and How They Fail"
- ^ a b "A non-rusting steel". New York Times. 31 January 1915.
- ^ "A Proposal to Establish the Shipwreck Half Moon as a State Underwater Archaeological Preserve". Bureau of Archaeological Research, Division of Historical Resources, Florida Department of State. May 2000. http://dhr.dos.state.fl.us/archaeology/underwater/preserves/HM_Prop3.pdf.
- ^ "ThyssenKrupp Nirosta: History". http://www.nirosta.de/History.22.0.html?&L=1. Retrieved 2007-08-13.
- ^ Scientific American Inventions and Discoveries, p. 380, Rodney P. Carlisle, John Wiley and Sons, 2004, ISBN 0471244104, ISBN 9780471244103
- ^ Sheffield Steel, ISBN 0-7509-2856-5
- ^ Cobb, Harold M. (2010). The History of Stainless Steel. ASM Internationa. p. 360. ISBN 1615030107.
- ^ "New Steel Alloy Is Rust Proof", December 1930, Popular Science
- ^ Ashby, Michael F.; & David R. H. Jones (1992) [1986]. "Chapter 12". Engineering Materials 2 (with corrections ed.). Oxford: Pergamon Press. pp. 119. ISBN 0-08-032532-7.
- ^ "What is Stainless Steel?". Nickel Institute. http://www.nickelinstitute.org/index.cfm/ci_id/11021.htm. Retrieved 2007-08-13.
- ^ What is Stainless Steel?
- ^ "Stainless Steel Bridge in Bilbao". Outokumpu. http://www.outokumpu.com/Industry-offerings/Architecture-Building-and-Construction/Case-Studies-Container/Bilbao-bridge/.
- ^ Gateway Arch Fact Sheet
- ^ "The Recycling of Stainless Steel ("Recycled Content" and "Input Composition" slides)" (Flash). International Stainless Steel Forum. 2006. http://www.worldstainless.org/ISSF/Files/Recycling08/Flash.html. Retrieved 2006-11-19.
- ^ ASTM A 387/ A387M - 06a Standard Specification for Pressure Vessel Plates, Alloy Steel, Chromium-Molybdenum
- ^ Material Properties Data: Marine Grade Stainless Steel
- ^ Shapeways: Stainless Steel
- ^ Material Properties Data: 3D Printing Steel
External links
- Animated presentation outlining the manufacture process by industry leaders Outokumpu
- Glossary of Stainless Steel Related Terms by Specialty Steel Supply
- Articles About Stainless Steel by International Stainless Steel Forum
- Stainless Steel Properties and Corrosion Resistance
- Comprehensive Information About Stainless Steel by The Stainless Steel Information Center
- Technical Library on Stainless Steel by BSSA
- Comprehensive Information About Metallurgy of Stainless Steel by Cambridge University
- Converter for Stainless Steel Standards
- Stainless Steel Composition
- Super Duplex Imperial Properties
- Super Duplex Metric Properties
- Comparison of foreign standards in stainless steel specifications
- Online Information Centre for Stainless Steel in Construction by the SCI.org of the UK
- Practical Guidelines for the Fabrication of Duplex Stainless Steels by IMOA
- Tables of Technical Properties by Euro Inox
- Guide to Stainless Steel Finishes by Euro Inox
- FAQ: Cleaning Stainless Steel in the Home by Euro Inox
- The Ferritic Solution. Properties, Advantages, Applications by ISSF
- How do you remove and prevent flash rust on stainless steel? Article about the preventions of flash rust
|
|||||||||||||||||||||||||||||||||||||||